Nanoparticle-Mediated Drug Delivery
pH-Responsive Hydrogels for the Oral Delivery of High Isoelectric Point Therapeutic Proteins
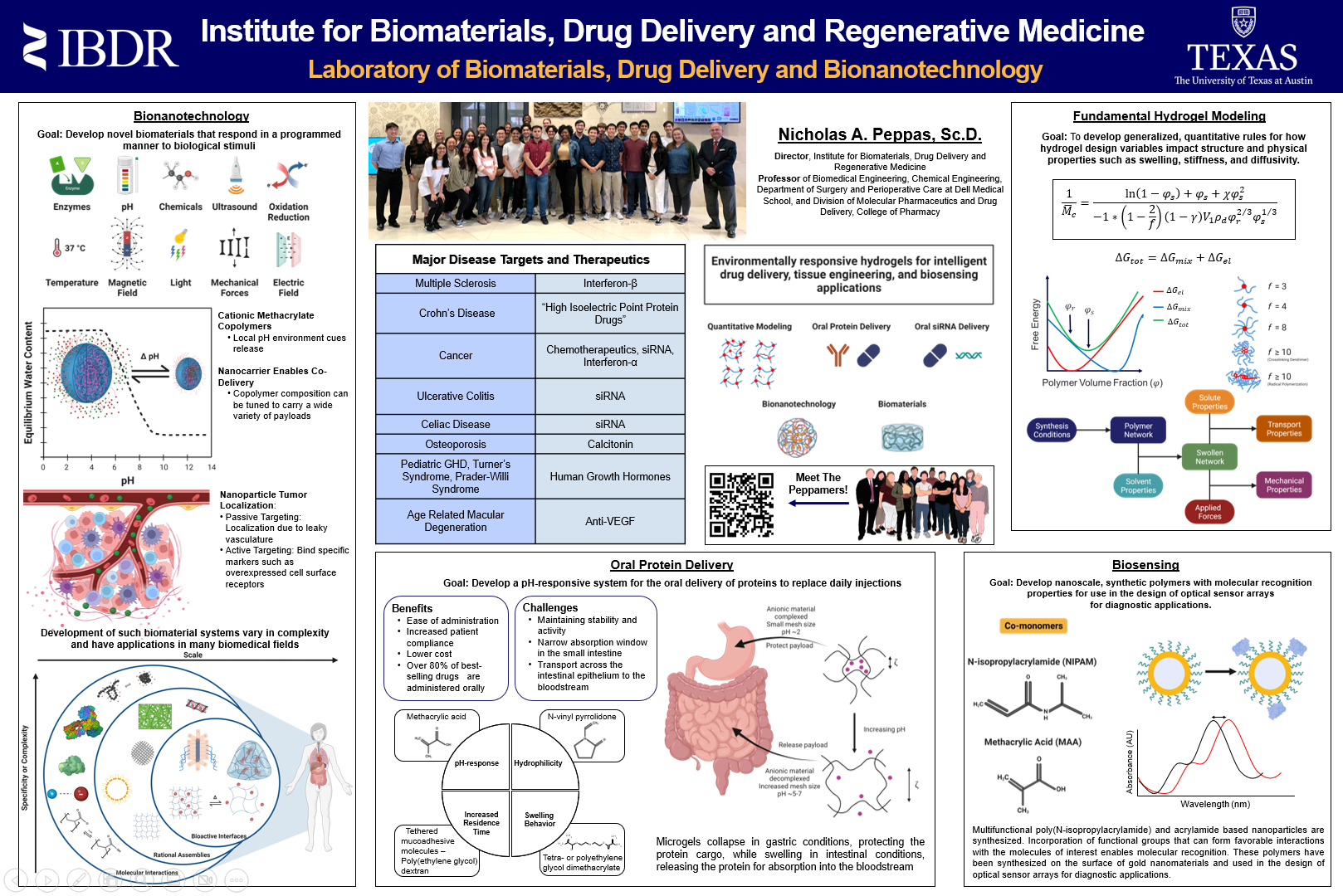
Protein therapeutics represent a rapidly growing pharmacological sector, but due to poor stability and large molecular weight, protein administration is almost exclusively limited to injection, which can be very painful and is often associated with fear and major side effects at the injection site. Achieving oral delivery is ideal and would almost certainly improve patient compliance but is fraught with challenges. Efforts to develop oral delivery platforms have been limited by the conditions in the gastrointestinal tract, which greatly reduce the activity of the proteins, and thus, their bioavailability is negligible if delivered in an unprotected state. Environmentally responsive polymer carriers, such as those that respond to changes in pH, have been explored for the development of an oral delivery platform for protein drugs. These pH-responsive carriers rely on charge interactions for the complexation behavior that facilitates protein loading and release. This characteristic presents a difficulty with proteins that exhibit a high isoelectric point, as the protein is likely to remain bound to the anionic hydrogel carrier at the pH of the small intestine instead of being repelled. This interaction must be overcome to allow protein release and thus, therapeutic efficacy. The aims of our work are to explore strategies for increasing the bioavailability of therapeutic proteins delivered via the oral route. To this end, copolymeric nanoparticle systems containing acrylamide and itaconic acid and crosslinked with N,N’-methylenebisacrylamide are synthesized via inverse emulsion polymerization. These particle systems are evaluated for oral protein delivery application.
Design of a Targeted Dual Hydrogel Delivery System for siRNA Delivery in Inflammatory Bowel Disease Therapy
Inflammatory Bowel Diseases (IBDs) are a group of chronic, relapsing conditions characterized by the inflammation of the gastrointestinal tract. As the disease is idiopathic in nature, the development of treatments with long-term efficacy has been challenging. RNA interference (RNAi) has emerged as a new approach to mitigate the aberrant cytokine signaling that is observed in IBDs. Through the use of small-interfering RNAs (siRNAs), undesirable genes can be silenced through the direct degradation of the mRNAs encoded by those genes. These siRNAs however need adequate carriers to ensure the delivery of these agents to the desired site of action. The optimal drug delivery carrier would ensure the siRNA payload is protected extracellularly and then release the siRNA into the cytosol for gene silencing purposes. In this project, we optimize functionalized hydrogel nanoparticle (nanogel) carriers for the targeted, intracellular delivery of siRNA to cells of the innate immune system. These nanogels exhibit pH-dependent properties such that they protect the siRNA payload extracellularly and release the cargo upon cellular internalization. This targeted approach, which promotes the uptake of the functionalized siRNA loaded nanogels into macrophages, could serve as a promising approach for more effective RNAi based therapies in IBDs.
In Vivo Modulation of Dendritic Cells by a Multi-Component Nanoscale System for Synergistic Immunotherapeutic Treatments
The American Cancer Society estimates colorectal cancer causing the second highest cancer-related deaths in 2020 due to an abysmal 14% 5-year relative survival rate when the cancer has metastasized, indicating a key unmet need for improved metastatic cancer therapeutic treatments. Immunotherapy has shown potential for improving cancer treatment due to reduced side effects from increased specificity and reduced chance for relapse due to immunological memory. Despite the growing popularity for immunotherapy, clinical translation for immunotherapeutic treatments have not seen as much success due to a lack in therapeutic efficacy to trigger an immune response for complete tumor eradication. Recent work involving nanoparticles for dendritic cell activation to trigger an immune response has lacked the ability for efficient cytosolic delivery, which is extremely important for cross presentation to activate CD8 killer T cells against exogenous antigens. In addition, the complex tumor microenvironment and immunosuppression mechanisms has rendered many treatments that only target a single pathway ineffective. In this work, we present a dual delivery platform for more effective immunotherapeutic treatments by combining intelligent pH responsive biomaterials for DC activation with targeted immune checkpoint blockade treatment to achieve a synergistic effect between multiple immune response phases.
Polycationic Nanogels for Targeted MicroRNA Delivery in the Treatment of Gioblastoma Multiforme
Glioblastoma multiforme (GBM) is one of the most aggressive forms of cancer boasting an abysmal 15-month survival-rate with current treatment options. For this reason, development of new therapeutic strategies is necessary. Recently, microRNA (miRNA) therapy has been developed as a means to suppress tumorigenesis in cancer patients. However, targeted miRNA delivery remains a barrier for further development and implementation of this therapeutic strategy. In this work, we propose developing a pH-responsive drug delivery platform for targeted miRNA delivery that will also bypass the highly selective blood-brain barrier for use in patients with GBM. By implementing a carrier-mediated transport system, we can increase miRNA stability and integrity by avoiding rapid degradation in the blood. Desirable carriers will remain collapsed in neutral and basic conditions and swell in the more acidic conditions that are characteristic of the tumor microenvironment.
Hydrogel Scaffolds for Regenerative Medicine
Distinguishing Mechanosensing and Diffusive Cytokine Signaling Effects on In Vitro Hematopoietic Stem Cell Culture
The hematopoietic stem cell (HSC) niche in bone marrow coordinates cell-cell signaling, cytokine signaling, and extracellular matrix signaling to maintain adult HSCs and promote differentiation to replenish blood cell counts or respond to immunological stress. Mimicking the HSC niche in vitro, both for HSC expansion and fundamental study of HSC behavior in healthy and diseased regimes, has proven an ongoing challenge due to the complexity of the natural environment. While it is clear that HSCs are affected both by the stiffness of the extracellular matrix and the diffusivity of solutes within the matrix, these two physical factors are closely correlated in typical hydrogel designs. With this project, we manipulate structural variables in hydrogel design with the goal to decouple stiffness and solute diffusivity. We then aim to use these hydrogels as scaffolds to investigate HSC responses to independent changes in stiffness and solute diffusivity.
Designing a System for the Controlled Delivery of Growth Factors for Bone Regeneration
In recent years, bone tissue engineering has emerged as a promising solution to the limitations of current gold standard treatment options for non-union bone fractures. Bone tissue engineering relies on a scaffold design that mimics the extracellular matrix, providing an architecture that guides the natural bone regeneration process. Incorporation of osteogenic growth factors, such as bone morphogenic proteins, into such scaffolds has been of particular interest due to their ability to enhance cell recruitment and promote osteogenesis; however, current growth factor delivery methods come with their own challenges. A key challenge is the inability to maintain the growth factors at the site of injury for the sustained period of time needed to achieve complete fracture healing. In addition, failure to stimulate endogenous repair mechanisms through the delivery of osteogenic factors alone can limit the size of regenerated bone tissue. The goal of this project is to develop a biomimetic system consisting of a biomaterial scaffold and polymeric nanoparticles to improve growth factor retention and provide controlled release of multiple growth factors involved in bone regeneration.
Protein-Recognitive Biosensors
Multifunctional Hydrogel-Coated Gold Nanoshells for the Detection of Sjögren’s Syndrome Protein Biomarkers
Autoimmune diseases put immense burdens on patients during diagnosis and treatments. Patients with Sjögren’s syndrome (SS), for example, experience recurring dry eyes and dry mouth from lymphocytic attacks on the lacrimal and salivary glands; however, treatment is further complicated in many cases by delayed diagnosis cause by the lack of clear indicators of the condition over other potential causes. There is an urgent need to develop a diagnostic method that can be applied for rapid and robust screening of symptomatic patients. Although clinical markers are often not present in the early stages of SS, proteomic research has identified variations in protein concentrations for SS compared to healthy controls. These biomarkers offer a promising opportunity for early detection; however, research in this area is challenged by the lack of disease specific markers and a multitude of competing proteins. The goal of this project is to develop a protein selective hydrogel coating on the surface of gold nanoshells as a biosensor for three key SS protein biomarkers that uses a multiplex sensor array and differential sensing approach to identify clinically relevant changes in concentration in lacrimal fluid that could enable early detection of SS.